SARS-CoV-2 Reduction
- Authors
- Dana Yee, M.D.
- Facility
- Innovative Bioanalysis, California
- Download
- Full Report
Plasma Air 600 Reduces SARS-CoV-2
PRODUCT BACKGROUND
Model tested was PA662. Applicable products include all models in the PA660 Series and PA600 Series.
The 660 series are needlepoint brush type ionizers producing an equal amount of positive and negative ions neutralizing harmful pollutants and odors. This self-balancing unit is installed at the fan inlet of an air handling unit, fan coil unit, PTAC, heat pump or a VRF ductless split system. The 660 series includes dry contacts which indicate ionizer functionality to a BAS (Building Automation System). This unit is UL2998 validated for zero ozone emissions.
OBJECTIVE
WellAir/Plasma Air supplied the Plasma Air PA662 for testing purposes to determine efficacy against viral pathogens. This study evaluated the effectiveness of the PA662 in its ability to reduce the viral strain referred to as SARS-CoV-2 Omicron within the air. Note, the PA600 Series and PA660 Series use the same ionization component.
TEST METHOD
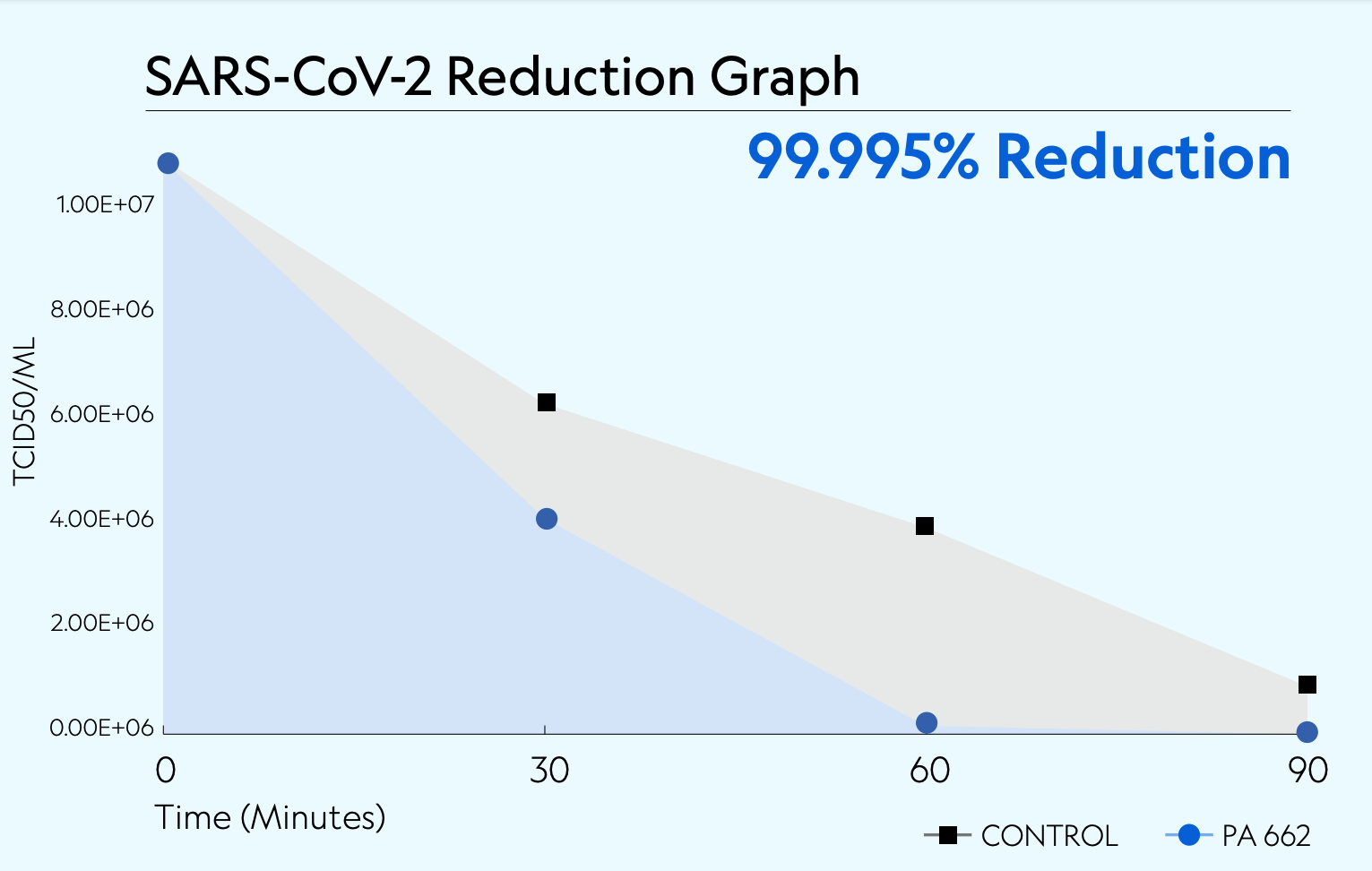
Testing was conducted in a sealed 20'x8'x8' chamber following BSL-3 standards. The temperature during testing was approximately 74 ±2°F (23.3 ±1.1°C), with a relative humidity of 41%. A 1.08 x 107 TCID50/mL of SARS-CoV-2 Omicron variant in viral media was nebulized into the chamber with mixing fans before collection. Air samples were collected at 30, 60, and 90 minutes after exposure.
Testing Layout
Testing was conducted in a sealed 20' × 8' × 8' chamber per Biosafety Level 3 (BSL3) standards. The overall dimensions of the test chamber provided a displacement volume of 1,280 ft3 (approximately 36,245 liters) of air. The chamber remained closed during testing, with no air entering or leaving the room. The chamber was equipped to create the necessary airflow to produce the required concentration of ions.* The temperature during testing was approximately 72 ±2°F (22.2 ±1.1°C), with a relative humidity of 37%. A 7.01 × 106 TCID50/mL of SARS-CoV-2 in viral media was nebulized into the chamber with mixing fans before collection. Air samples were collected at 30, 60, and 90 minutes after exposure.
Control Results
Control testing was conducted without the device operating in duplicate, and samples were taken at the corresponding time points used for the challenge. The results displayed a natural viability loss over time in the chamber and were used as a comparative baseline to calculate viral reduction.
CONCLUSION
The Plasma Air PA662 demonstrated an overall capability in reducing aerosolized SARS-CoV-2 Omicron viruses at each time point faster than the natural viability loss rates. After 30 minutes of operation, a 62.401% gross reduction was observed and increased with longer exposure time, as shown by the 99.995% (5 log) reduction achieved after 90 minutes.